BioRoot™ RCS
Endo sealer or biological filler?
Reading 12 min.
The historical aspects
Root canal obturation is necessary to fill the dead space left behind when the pulp is lost. Root canal treatment methodologies are very old and have changed very little over the years. The root canal obturation is usually undertaken using a solid cone and a sealer. Initially it was used as a single cone together with root canal sealer, then the techniques evolved to lateral condensation and warm vertical compaction to enhance the three dimensional quality of the root canal filling (1).
The core acts as a piston on the flowable sealer, causing it to spread, fill voids and to wet and attach to the instrumented dentin wall. It is the sealer that comes into contact with the dentine and periodontal tissues. It is thus important that the sealer possesses the ideal material properties as outlined by Grossman (2).
The three primary functions of a root filling are the sealing against ingrowth of bacteria from the oral cavity, entombment of remaining microorganisms and complete obturation at a microscopic level to prevent stagnant fluid from accumulating and serving as nutrients for bacteria from any source (3). For this purpose the gutta-percha solid cone-sealer association has had considerable success and hermetic seal is provided by warm vertically compacted guttapercha with a selection of sealers which interact with the dentine walls forming sealer tags. The epoxy resin-based root canal sealers have been termed the gold standard for sealer cements as they serve this purpose very well and provide an hermetic seal.
The hydraulic filler obturation
BioRoot™ RCS (Septodont, Saint-Maur-desFossés, France) is a hydraulic cement which is presented as a powder composed of tricalcium silicate, and zirconium oxide and a liquid which is mainly water-based with additions of calcium chloride and a water soluble polymer. These additives enhance the physical characteristics of the material. This specific formulation imparts definitive characteristics to this hydraulic material. These characteristics include the following.
Low levels of trace elements
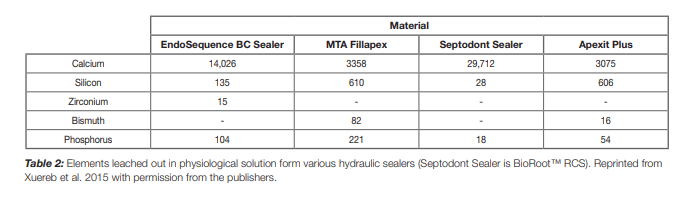
Most of the materials which are known to be based on tricalcium silicate are made of Portland cement. Portland cement is a material used in the construction industry and thus it is manufactured from natural minerals. Furthermore to keep the costs down secondary fuels which are usually wastes are used for burning the cement. This results in the inclusion of trace elements in the cement and these are leached in solution when in clinical use (4-6). BioRoot™ RCS is the only material which is made entirely of pure tricalcium silicate cement with no other cementitious additions (Table 1). This property is important not only to avoid the trace minerals but also because the active part of the material is the tricalcium silicate. Portland cement only has 68% tricalcium silicate (7). Thus all the properties attributed to the tricalcium silicate namely the formation of calcium hydroxide which is responsible for the biomineralization, bone and hard tissue formation and antimicrobial properties will be less evident with the use of Portland cement. In fact BioRoot™ RCS releases double the amount leached by Endosequence BC sealer and ten times as much as calcium ions released by MTA Fillapex (Table 2) for the same time periods under the same conditions (8).
Three Porland cement-based materials including MTA Angelus, MTA Fillapex and Theracal LC have been tested to observe whether the presence of these materials in the dental extraction socket of an in vivo model would affect the level of aluminum in the plasma and liver. Traces of aluminium have been detected in the plasma and liver of test animals (9). Furthermore an aluminium peak was observed in the brain tissues of test animals after 7 days from implantation in MTA Angelus and after 60 days in Theracal and MTA Fillapex. Oxidative stress was induced and antioxidant enzymes were transiently upregulated (10). High levels of aluminium in contact with human tissues has been linked with Alzheimer’s disease (11). On contrary, BioRoot™ RCS as a pure tricalcium silicate cement does not contain any tricalcium aluminate phase. There is no leaching of aluminium when BioRoot™ RCS in direct contact with the patient tissues. So this material will not lead to any toxic deposition of trace elements.
The use of inert radiopacifier
BioRoot™ RCS has zirconium oxide radiopacifier. The zirconium oxide is stable (Table 2) and imparts the necessary radiopacity to the material (Figure 1)(8). Since there is no leaching the radiopacity will be stable when in clinical use. BioRoot™ RCS is easy to detect on a radiograph post-operatively thus makes the assessment of obturation easier. BioRoot™ RCS does not use bismuth oxide as a radiopacifier. Bismuth oxide has been shown to result in tooth discolouration when in contact with sodium hypochlorite (12) which is used as irrigating solution for all endodontic cases.
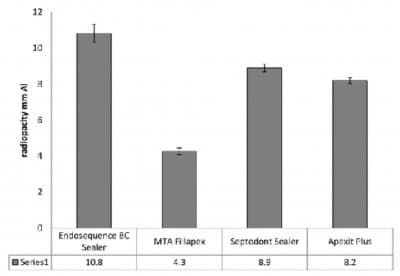
Fig. 1: Radiopacity of various sealers. (Septodont Sealer is BioRoot™ RCS). Reprinted from Xuereb et al. 2015 with permission from the publishers.
Enhanced antimicrobial properties
The success of endodontic therapy depends on the elimination of microbes and prevention of bacterial recolonisation of the root canal. BioRoot™ RCS leaches high levels of calcium in solution (Table 2) thus maintains a high pH. It exhibits optimal antimicrobial properties as indicated by the elimination of microorganisms in the dental tubules (Figure 2). BioRoot™ RCS is effective in elimination of microorganisms even when water is used as final irrigating solution (12) and its activity is enhanced when using ethylene diamine tetracetic acid (EDTA) irrigating solution.
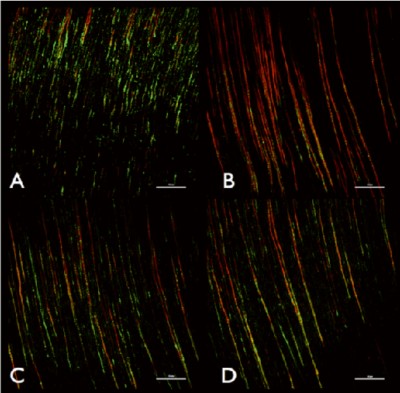
Fig. 2: Representative confocal laser scanning microscope images of (A) control group irrigated with EDTA, (B) BioRoot™ RCS after EDTA, (C) MTA Fillapex after EDTA and (D) AH Plus after EDTA. Bars represent 50 mm. The red colour signifies dead microbes. Reprinted from Arias Moliz & Camilleri 2016 with permission from publisher.
Biological seal
BioRoot™ RCS interacts with the dentine along the root canal wall and forms a hybrid layer along the dentine which is rich in mineral (Figure 3). The bonding of BioRoot™ RCS is postulated to be chemical in nature as opposed to the sealer tags reported for resin-based sealers (13). This strong bond helps with the sealer stability. This is coupled with the high antimicrobial characteristics thus making this material superior to other sealer types. BioRoot™ RCS is well tolerated by the periodontal tissues (14-16) and any extrusions will not be deleterious to the clinical success.
Obturation method
BioRoot™ RCS should be used with cold obturation techniques. The heat generated during warm vertical compaction will lead to the evaporation of water from the sealer thus modifying the flow and film thickness of the material (17). More recently single cone obturation techniques are being suggested for hydraulic sealers. Dentinal tubule penetration occurs independent of which obturation techniques technique is used (18, 19). If the solid cone is matched to the size of the preparation, the single cone obturation technique provides similar obturation quality to the warm vertical compaction (20). The retreatability of BioRoot™ RCS sealer used in conjunction with gutta-percha in single cone obturation technique was better compared to AH Plus as less sealer remnants and shorter retreatment times were observed (21).
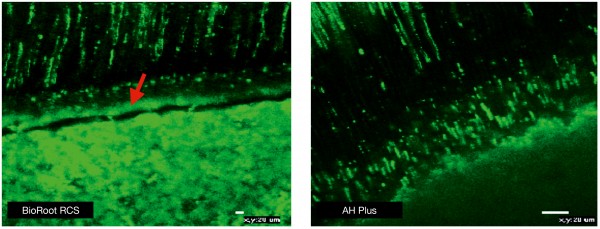
Fig 3: Interfacial characteristics for BioRoot™ RCS showing mineral rich layer at the interface (marked with arrow) and sealer tags as opposed to AH Plus which exhibits only the sealer tags. Materials mixed with Fluorescein dye to be viewed under confocal laser microscope at excitation/emission wavelength of 494/518 nm. Reprinted from Viapiana et al. 2016 with permission from publisher.
Conclusion
The BioRoot™ RCS is a hydraulic sealer that allows simple and effective root canal obturation. The material is non-toxic and can be used in conjunction with solid cones in a single cone obturation technique. This method is easy to use and more cost-effective no special armamentarium is required. The success of the obturation lies in the antimicrobial activity and the biological seal rather than the hermetic seal reported for classical sealers. The BioRoot™ RCS can be considered more as a filler used in conjunction with a solid cone.
1. Schilder H. Filling root canals in three dimensions. Dent Clin North Am. 1967.
2. Grossman LI. Endodontic Practice. Philadelphia: Lea & Febiger.1978
3. Sundqvist G, Figdor D. Endodontic treatment of apical periodontitis. In: Ørstavik D, Pitt Ford TR, eds.
Essential Endodontology. Prevention and Treatment of Apical Periodontitis. Oxford: Blackwell, 1998.
4. Schembri M, Peplow G, Camilleri J. Analyses of heavy metals in mineral trioxide aggregate and Portland
cement. J Endod. 2010;36(7):1210-5.
5. Chang SW, Shon WJ, Lee W, Kum KY, Baek SH, Bae KS. Analysis of heavy metal contents in gray and
white MTA and 2 kinds of Portland cement: a preliminary study. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 2010;109(4):642-6.
6. Monteiro Bramante C, Demarchi AC, de Moraes IG, Bernadineli N, Garcia RB, Spångberg LS, Duarte MA.
Presence of arsenic in different types of MTA and white and gray Portland cement.Oral Surg Oral Med Oral
Pathol Oral Radiol Endod. 2008;106(6):909-13.
7. Camilleri J. Characterization and hydration kinetics of tricalcium silicate cement for use as a dental
biomaterial. Dent Mater. 2011;27(8):836-44.
8. Xuereb M, Vella P, Damidot D, Sammut CV, Camilleri J. In situ assessment of the setting of tricalcium
silicate-based sealers using a dentin pressure model. J Endod. 2015;41(1):111-24.
9. Demirkaya K, Can Demirdöğen B, Öncel Torun Z, Erdem O, Çetinkaya S, Akay C (2016). In vivo evaluation
of the effects of hydraulic calcium silicate dental cements on plasma and liver aluminium levels in rats. Eur
J Oral Sci. 124(1):75-81.
10. Demirkaya K, Demirdöğen BC, Torun ZÖ, Erdem O, Çırak E, Tunca YM (2016). Brain aluminium
accumulation and oxidative stress in the presence of calcium silicate dental cements. Hum Exp Toxicol.
pii: 0960327116679713.
11. Forbes WF, Gentleman JF. Risk factors, causality, and policy initiatives: the case of aluminum and mental
impairment. Exp Gerontol 1998:33:141–54.
12. Arias-Moliz MT, Camilleri J. The effect of the final irrigant on the antimicrobial activity of root canal sealers.
J Dent. 2016;52:30-6.
13. Viapiana R, Moinzadeh AT, Camilleri L, Wesselink PR, Tanomaru Filho M, Camilleri J. Porosity and sealing
ability of root fillings with gutta-percha and BioRoot™ RCS or AH Plus sealers. Evaluation by three ex vivo
methods. Int Endod J. 2016;49(8):774-82.
14. Collado-González M, García-Bernal D, Oñate-Sánchez RE, Ortolani-Seltenerich PS, Lozano A, Forner L,
Elena C, Rodríguez-Lozano FJ. Biocompatibility of three new calcium silicate-based endodontic sealers
on human periodontal ligament stem cells. Int Endod J. 2016 Sep 26. doi: 10.1111/iej.12703. [Epub ahead
of print].
15. Poggio C, Riva P, Chiesa M, Colombo M, Pietrocola G. Comparative cytotoxicity evaluation of eight root
canal sealers. J Clin Exp Dent. 2017;1;9(4):e574-e578.
16. Camps J, Jeanneau C, El Ayachi I, Laurent P, About I. Bioactivity of a Calcium Silicate- based Endodontic
Cement (BioRoot™ RCS): Interactions with Human Periodontal Ligament Cells In Vitro. J Endod.
2015;41(9):1469-73.
17. Camilleri J. Sealers and warm gutta-percha obturation techniques. J Endod. 2015;41(1):72-8.
18. Jeong JW, DeGraft-Johnson A, Dorn SO, Di Fiore PM. Dentinal Tubule Penetration of a Calcium Silicatebased Root Canal Sealer with Different Obturation Methods. J Endod. 2017;43(4):633-637.
19. McMichael GE, Primus CM, Opperman LA. Dentinal Tubule Penetration of Tricalcium Silicate Sealers. J
Endod. 2016;42(4):632-6.
20. Alshehri M, Alamri HM, Alshwaimi E, Kujan O. Micro-computed tomographic assessment of quality of
obturation in the apical third with continuous wave vertical compaction and single match taper sized cone
obturation techniques. Scanning. 2016;38(4):352-6.
21. Donnermeyer D, Bunne C, Schäfer E, Dammaschke T. Retreatability of three calcium silicate-containing
sealers and one epoxy resin-based root canal sealer with four different root canal instruments. Clin Oral
Investig. 2017 Jun 22. doi: 10.1007/ s00784-017-2156-5. [Epub ahead of print]



